Intro
Discover 5 surprising drug name facts, exploring pharmaceutical naming conventions, generic names, and brand name strategies, revealing the science behind medication monikers.
The world of pharmaceuticals is vast and complex, with thousands of drugs available to treat a wide range of conditions. One aspect of the pharmaceutical industry that is often overlooked is the process of naming drugs. Drug names are carefully chosen to be unique, memorable, and easy to pronounce. In this article, we will delve into the fascinating world of drug names, exploring the history, regulations, and cultural influences that shape the naming of medications.
The process of naming a drug is a meticulous one, involving a combination of creativity, research, and regulatory compliance. Pharmaceutical companies must ensure that their drug names are distinct from existing medications, while also being easy to remember and pronounce. This is crucial, as a confusing or difficult-to-pronounce name can lead to medication errors, which can have serious consequences for patients. Furthermore, drug names must also comply with regulatory requirements, such as those set by the Food and Drug Administration (FDA) in the United States.
The importance of drug names cannot be overstated, as they play a critical role in patient safety, medication adherence, and the overall effectiveness of treatment. A well-chosen drug name can help to establish a brand identity, build trust with patients and healthcare professionals, and differentiate a medication from its competitors. In contrast, a poorly chosen name can lead to confusion, errors, and a negative perception of the medication. As we explore the world of drug names, we will examine the key factors that influence the naming process, including regulatory requirements, cultural influences, and marketing strategies.
History of Drug Naming

Regulatory Requirements
The regulatory requirements for drug naming vary by country and region, but most agencies follow similar guidelines. In the United States, the FDA requires that drug names be unique, not easily confused with existing medications, and easy to pronounce. The agency also recommends that drug names be descriptive, indicating the medication's use, ingredients, or mechanism of action. For example, the drug name "Lipitor" suggests that the medication is used to lower lipids, or fats, in the blood.Cultural Influences on Drug Naming

Marketing Strategies
Marketing strategies also play a critical role in the naming of medications, as pharmaceutical companies seek to create a brand identity and differentiate their products from competitors. Drug names can be used to convey a sense of innovation, efficacy, or safety, and can help to establish a medication as a leader in its class. For example, the drug name "Viagra" was chosen for its unique sound and spelling, as well as its association with the concept of "vitality" and " vigor."Types of Drug Names

Generic Names
Generic names are the official names of medications, used to identify the active ingredient or ingredients in a medication. Generic names are usually descriptive, indicating the medication's use, ingredients, or mechanism of action. For example, the generic name "acetaminophen" indicates that the medication is a pain reliever and fever reducer.Benefits of Unique Drug Names
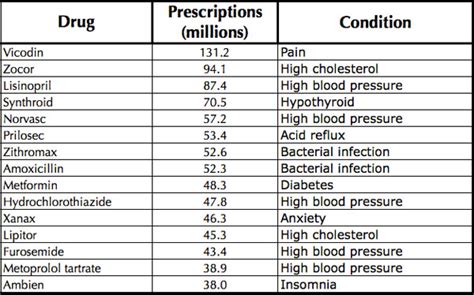
Challenges of Drug Naming
Despite the importance of unique drug names, the process of naming medications can be challenging. Pharmaceutical companies must balance the need for a unique and memorable name with the need for a name that is easy to use and pronounce. Additionally, regulatory agencies may reject proposed drug names if they are deemed to be confusing or misleading. The increasing number of medications on the market also makes it more difficult to choose a unique and distinctive name.Future of Drug Naming
Emerging Trends
Several emerging trends are likely to influence the future of drug naming, including the increasing use of biosimilars, the growth of personalized medicine, and the rising importance of patient-centered care. Biosimilars, which are biological medications that are similar to existing products, will require unique names that distinguish them from their reference products. Personalized medicine, which involves the use of targeted therapies and tailored treatment approaches, may require more descriptive and informative names that reflect the medication's specific use and mechanism of action.Conclusion and Next Steps

We invite you to share your thoughts and comments on the topic of drug naming, and to explore the many resources and references available on this subject. Whether you are a healthcare professional, a patient, or simply interested in the pharmaceutical industry, we hope that this article has provided you with a deeper understanding of the complex and fascinating world of drug names.
What is the purpose of drug naming?
+The purpose of drug naming is to provide a unique and descriptive name for a medication, which can help to promote patient safety, medication adherence, and effective treatment.
How are drug names chosen?
+Drug names are chosen through a careful process of research, creativity, and regulatory compliance, involving the consideration of factors such as uniqueness, memorability, and ease of use.
What are the benefits of unique drug names?
+Unique drug names offer several benefits, including improved patient safety, reduced medication errors, and enhanced brand recognition, which can help to establish a medication as a leader in its class.
