Intro
Discover what hydrochloride is, its uses, and effects. Learn about hydrochloride salts, acid, and compounds in chemistry, pharmacology, and medicine, including hydrochloric acid and sodium hydrochloride.
Hydrochloride is a term used in chemistry to describe a type of salt that is formed when a base, typically an amine or an alkali, reacts with hydrochloric acid. The resulting compound is a hydrochloride salt, which is often used in various industries, including pharmaceuticals, agriculture, and manufacturing. Hydrochloride salts are commonly used as intermediates in the production of other chemicals, as well as in the formulation of various products, such as medications, fertilizers, and dyes.
The importance of hydrochloride cannot be overstated, as it plays a crucial role in many industrial and commercial applications. For instance, in the pharmaceutical industry, hydrochloride salts are used to formulate medications that are more stable, soluble, and bioavailable. In agriculture, hydrochloride salts are used as fertilizers to promote plant growth and increase crop yields. Additionally, hydrochloride salts are used in the manufacturing of various products, such as plastics, textiles, and paper.
The use of hydrochloride salts has been increasing over the years, driven by the growing demand for these compounds in various industries. As a result, there is a need for a comprehensive understanding of hydrochloride, including its properties, applications, and benefits. In this article, we will delve into the world of hydrochloride, exploring its definition, types, applications, and benefits, as well as its potential drawbacks and future prospects.
Definition and Properties of Hydrochloride

Hydrochloride is a type of salt that is formed when a base, typically an amine or an alkali, reacts with hydrochloric acid. The resulting compound is a hydrochloride salt, which is characterized by its unique properties, such as its high solubility in water, its stability, and its reactivity. Hydrochloride salts are often white or colorless crystals, with a salty or bitter taste. They are highly soluble in water, which makes them useful in various applications, such as in the formulation of medications and fertilizers.
Types of Hydrochloride
There are several types of hydrochloride salts, each with its unique properties and applications. Some of the most common types of hydrochloride salts include: * Ammonium hydrochloride: This is a type of hydrochloride salt that is formed when ammonia reacts with hydrochloric acid. It is commonly used in the production of fertilizers, dyes, and pharmaceuticals. * Sodium hydrochloride: This is a type of hydrochloride salt that is formed when sodium hydroxide reacts with hydrochloric acid. It is commonly used in the production of soap, paper, and textiles. * Calcium hydrochloride: This is a type of hydrochloride salt that is formed when calcium carbonate reacts with hydrochloric acid. It is commonly used in the production of cement, concrete, and mortar.Applications of Hydrochloride
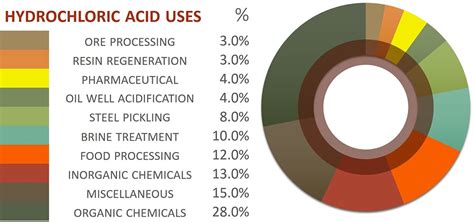
Hydrochloride salts have a wide range of applications in various industries, including pharmaceuticals, agriculture, and manufacturing. Some of the most common applications of hydrochloride salts include:
- Pharmaceuticals: Hydrochloride salts are used in the formulation of medications, such as antihistamines, antacids, and anesthetics.
- Agriculture: Hydrochloride salts are used as fertilizers to promote plant growth and increase crop yields.
- Manufacturing: Hydrochloride salts are used in the production of various products, such as plastics, textiles, and paper.
Benefits of Hydrochloride
The use of hydrochloride salts has several benefits, including: * Improved solubility: Hydrochloride salts are highly soluble in water, which makes them useful in various applications, such as in the formulation of medications and fertilizers. * Increased stability: Hydrochloride salts are more stable than their parent compounds, which makes them useful in various applications, such as in the production of pharmaceuticals and agricultural products. * Enhanced reactivity: Hydrochloride salts are highly reactive, which makes them useful in various applications, such as in the production of plastics and textiles.Working Mechanism of Hydrochloride
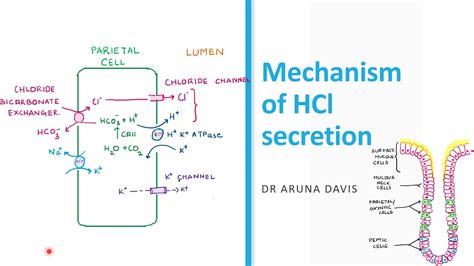
The working mechanism of hydrochloride salts involves the reaction of a base, typically an amine or an alkali, with hydrochloric acid. The resulting compound is a hydrochloride salt, which is characterized by its unique properties, such as its high solubility in water, its stability, and its reactivity. The reaction involves the transfer of a proton from the hydrochloric acid to the base, resulting in the formation of a hydrochloride salt.
Steps Involved in the Production of Hydrochloride
The production of hydrochloride salts involves several steps, including: 1. Synthesis: The base, typically an amine or an alkali, is synthesized through a series of chemical reactions. 2. Reaction: The base is then reacted with hydrochloric acid to form a hydrochloride salt. 3. Purification: The resulting hydrochloride salt is then purified through various methods, such as recrystallization and distillation. 4. Formulation: The purified hydrochloride salt is then formulated into various products, such as medications, fertilizers, and dyes.Practical Examples of Hydrochloride

There are several practical examples of hydrochloride salts, including:
- Ammonium hydrochloride: This is a type of hydrochloride salt that is commonly used in the production of fertilizers, dyes, and pharmaceuticals.
- Sodium hydrochloride: This is a type of hydrochloride salt that is commonly used in the production of soap, paper, and textiles.
- Calcium hydrochloride: This is a type of hydrochloride salt that is commonly used in the production of cement, concrete, and mortar.
Statistical Data on Hydrochloride
The use of hydrochloride salts has been increasing over the years, driven by the growing demand for these compounds in various industries. According to statistical data, the global market for hydrochloride salts is expected to grow at a rate of 5% per annum, driven by the increasing demand for these compounds in the pharmaceutical, agricultural, and manufacturing industries.Future Prospects of Hydrochloride
The future prospects of hydrochloride salts are promising, driven by the growing demand for these compounds in various industries. The increasing use of hydrochloride salts in the pharmaceutical, agricultural, and manufacturing industries is expected to drive the growth of the global market for these compounds. Additionally, the development of new technologies and applications for hydrochloride salts is expected to further drive the growth of the market.
Challenges and Opportunities
Despite the promising future prospects of hydrochloride salts, there are several challenges and opportunities that need to be addressed. Some of the challenges include: * Environmental concerns: The production and use of hydrochloride salts can have environmental impacts, such as air and water pollution. * Health concerns: The use of hydrochloride salts can have health impacts, such as respiratory problems and skin irritation. * Competition: The market for hydrochloride salts is highly competitive, with several players competing for market share.On the other hand, there are several opportunities that need to be explored, including:
- Developing new applications: There is a need to develop new applications for hydrochloride salts, such as in the production of renewable energy and biodegradable products.
- Improving production processes: There is a need to improve the production processes for hydrochloride salts, such as by reducing energy consumption and waste generation.
- Increasing market share: There is a need to increase market share, such as by developing new products and services that meet the needs of customers.
What is hydrochloride?
+Hydrochloride is a type of salt that is formed when a base, typically an amine or an alkali, reacts with hydrochloric acid.
What are the applications of hydrochloride?
+Hydrochloride salts have a wide range of applications in various industries, including pharmaceuticals, agriculture, and manufacturing.
What are the benefits of hydrochloride?
+The use of hydrochloride salts has several benefits, including improved solubility, increased stability, and enhanced reactivity.
In conclusion, hydrochloride is a versatile compound with a wide range of applications in various industries. Its unique properties, such as its high solubility in water, its stability, and its reactivity, make it a valuable compound in the production of various products, such as medications, fertilizers, and dyes. As the demand for hydrochloride salts continues to grow, it is essential to address the challenges and opportunities associated with its production and use. We invite readers to comment, share this article, or take specific actions to learn more about hydrochloride and its applications.
