Intro
Discover how alginate meets calcium in 5 innovative ways, exploring its applications in food, pharmaceuticals, and dentistry, highlighting its gel-like properties, and showcasing its role in wound care, dental impressions, and calcium supplementation.
The interaction between alginate and calcium is a fundamental aspect of various industrial, biomedical, and culinary applications. Alginate, a naturally occurring polymer derived from brown seaweed, has unique properties that make it an ideal component in numerous products, from food to pharmaceuticals. Its ability to react with calcium ions is particularly significant, as this reaction is crucial for the formation of gels, films, and other structures that are essential in these applications. Understanding how alginate meets calcium and the consequences of their interaction is vital for optimizing its use across different sectors.
The versatility of alginate is largely attributed to its chemical structure, which includes carboxyl groups that can bind with calcium ions. This binding capability is what allows alginate to form strong, yet flexible, gels and membranes when exposed to calcium-rich environments. The applications of this alginate-calcium interaction are diverse, ranging from the production of desserts and beverages in the food industry, to wound dressings and drug delivery systems in the biomedical field. As research continues to uncover new properties and potential uses of alginate, its importance in both traditional and innovative applications is becoming increasingly evident.
The significance of the alginate-calcium interaction extends beyond its practical uses, as it also plays a critical role in the natural world. In marine environments, alginate produced by seaweeds interacts with calcium and other ions, influencing the chemical composition of seawater and the formation of marine sediments. This ecological aspect highlights the interconnectedness of biological and chemical processes in nature and underscores the value of studying these interactions for a deeper understanding of both natural and industrial systems.
Introduction to Alginate and Calcium Interaction

Chemical Basis of Alginate-Calcium Interaction
The chemical basis of the alginate-calcium interaction is rooted in the structure of the alginate molecule. Alginate is a polysaccharide composed of mannuronic and guluronic acid residues, arranged in a block-like pattern along the polymer chain. The guluronic acid blocks are particularly significant for the calcium binding capability of alginate, as they contain carboxyl groups that can chelate calcium ions. This chelation process is what initiates the cross-linking of alginate chains, resulting in the formation of a gel or solid structure.Applications of Alginate-Calcium Interaction
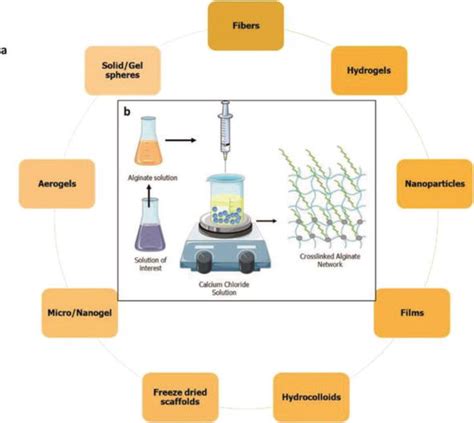
Biomedical Applications
In the biomedical field, the alginate-calcium interaction is utilized in the development of wound dressings, drug delivery systems, and tissue engineering scaffolds. Alginate's biocompatibility, coupled with its ability to form gels and membranes in response to calcium ions, makes it an ideal material for these applications. For instance, alginate-based wound dressings can promote wound healing by creating a moist environment that fosters tissue regeneration, while also protecting the wound from bacterial infections.Practical Examples of Alginate-Calcium Interaction
Steps for Utilizing Alginate-Calcium Interaction
To utilize the alginate-calcium interaction in a practical application, several steps can be followed: - **Selection of Alginate Type**: Choosing the appropriate type of alginate, based on its mannuronic to guluronic acid ratio, is crucial for achieving the desired properties in the final product. - **Calcium Ion Source**: Selecting a suitable source of calcium ions, such as calcium chloride or calcium carbonate, and determining the optimal concentration for the specific application. - **Mixing and Gelation**: Mixing the alginate with water and then introducing the calcium ions to initiate gelation, controlling factors such as temperature and mixing time to achieve the desired texture and consistency. - **Product Formation**: Shaping the gel or solid into the desired form, which could range from pouring into molds for desserts to applying as a layer in wound dressings.Benefits and Challenges of Alginate-Calcium Interaction

Future Perspectives
Looking to the future, the alginate-calcium interaction is expected to play an increasingly important role in the development of innovative materials and products. Advances in understanding the chemical and physical properties of alginate, as well as improvements in manufacturing technologies, are likely to expand its applications into new areas. The potential for alginate to contribute to sustainable and environmentally friendly products, due to its natural origin and biodegradability, is particularly noteworthy.Statistical Data and Research Findings

Conclusion and Recommendations
In conclusion, the interaction between alginate and calcium is a complex and highly useful phenomenon that underpins a wide range of industrial, biomedical, and culinary applications. By understanding the chemical basis of this interaction and optimizing its use, researchers and manufacturers can develop innovative products with tailored properties. Recommendations for future work include further investigation into the effects of different factors on the alginate-calcium interaction, as well as exploration of new applications that can leverage the unique properties of alginate.What is the primary mechanism behind the alginate-calcium interaction?
+The primary mechanism involves the ionic cross-linking of alginate chains by calcium ions, leading to the formation of a three-dimensional network.
What are some common applications of the alginate-calcium interaction?
+Common applications include the production of desserts and beverages in the food industry, wound dressings and drug delivery systems in the biomedical field, and tissue engineering scaffolds.
How can the properties of alginate-calcium products be controlled?
+The properties can be controlled by adjusting the concentration of alginate and calcium ions, as well as factors such as temperature, pH, and the presence of other ions.
We hope this comprehensive overview has provided valuable insights into the fascinating world of alginate and its interaction with calcium. Whether you're a researcher, manufacturer, or simply someone interested in the science behind everyday products, understanding this interaction can open up new avenues for innovation and discovery. Feel free to share your thoughts, ask questions, or explore further into the many applications and potential of alginate and calcium.
